Users, which foresee to access after 15th March 2017, of Spanish genetic resources from wild taxa whose activities involves the utilization of genetic resources. In this context, ‘utilization of genetic resources’ means to conduct research and development on the genetic and/or biochemical composition of genetic resources, including through the application of biotechnology as defined in the Law 42/2007, of 13 December, on natural heritage and biodiversity.
The European Commission is preparing several sectorial guidance documents on the interpretation of the term utilization within the scope of the Regulation (EU) No 511/2014, which are foreseen to be published soon. It is recommended that users consider these guidelines to establish whether activities carried out with genetic resources constitute ‘utilization’, and therefore fall within the scope of this bylaw.
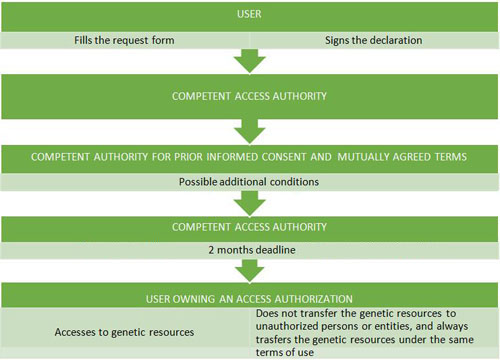
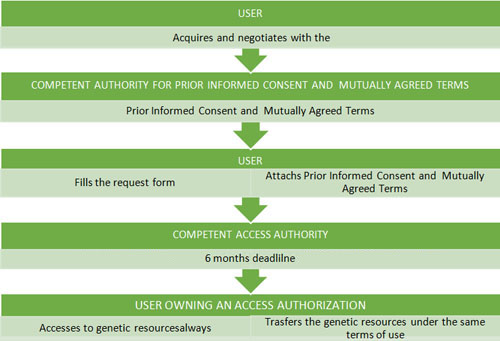
The bylaw 124/2017, of 24 February, regulates the access to genetics resources from wild taxa in situ, i.e. in their natural habitats, and ex situ, i.e. outside their natural habitats.
Access to the following Spanish genetics resources for their utilization does not fall in the scope of the bylaw 124/2017, of 24 February
- Plant genetic resources for agriculture and food regulated by the Law 30/2006, of 26 July, on seeds and plants from garden center and plant genetic resources.
- Fishery resources regulated by the Law 3/2001, of 26 March, on state maritime fishery.
- Zoogenetic resources for agriculture and food.
Additionally, access authorization is not necessary when:
- Users access to Spanish genetic resources for exclusively taxonomic purposes*, according to the definition of ‘exclusively taxonomic purposes’ in article 2.3 of the bylaw 124/2017, of 24 February. These genetic resources may only be transferred to subsequent users under the same terms they were accessed, that is with exclusively taxonomic purposes. Otherwise, an access authorization is required.
- Collection and preservation of samples in germplasm banks or ex situ collections for exclusively conservation purposes.
- Users that carry out activities of production and commercialization of seeds and forest plants regulated by the Bylaw 289/2003, of 7 March, on commercialization of forest breeding material, as long as the use of these genetic resources does not constitute utilization and the user do not transfer them to subsequent users for different uses.
*Exclusively taxonomic purposes (definition in Art. 2.3. Spanish ABS bylaw): Application of principles and methods for identification, delimitation and classification of living beings, which requires the study of their phylogenetic relationships as well as the evolutionary and ecological processes that have generated biodiversity using morphological, physiological, genetics, behavioral and environmental data.